Global economies are moving towards a larger use of renewable energy sources, in an effort of reducing fossil fuel consumption and the related global warming issues. However, renewables are intermittent in nature, which can be mitigated by chemical storage of the renewable energy. Hydrogen is considered one of the most suitable forms of chemical storage, since it can be produced by electrolysis of water, and its oxidation only involves the emission of water, thus forming a sustainable cycle. The most promising and safe form of hydrogen storage is the use of solid-state substrates, allowing the storage in favorable and safe pressure and temperature conditions. The use of graphene in solid-state hydrogen storage applications is object of extensive research work, motivated by the advantageous physiochemical properties of the material: graphene is lightweight, cheap, inert, and can be functionalized, all desirable properties for a storage material. However, the 2D nature of graphene makes hydrogen storage devices based on pristine flat graphene prohibitively large.
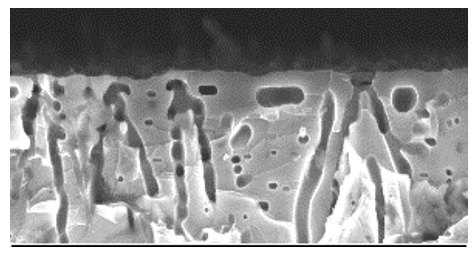
Thus, we have developed a novel 3D graphene structure suitable for hydrogen storage. Epitaxial graphene is grown on the (0001) surface of 4H-SiC wafers that have been porousified via photoelectrochemical etching, resulting in an extremely large surface area in a native 3D backbone. The figure shows SEM images of the new material. The total surface of this porous material is about 200 times larger than the top surface. Moreover, the new material preserves the outstanding properties of 2D graphene, thus allowing to accommodate a large graphene surface in a compact structure. We have studied the interactions of hydrogen with this material and obtained evidence for a catalytic splitting of hydrogen molecules by the bare material without any functionalization. Now experiments are ongoing to functionalize the 3D graphene arrangement with metal nanoparticles, suitable for hydrogen storage applications. In perspective, this porous material paves the way to applications in other fields such as sensors, electrodes for water splitting devices, and PEM fuel cells.
For more details, see http://web.nano.cnr.it/heun/research/graphene/.

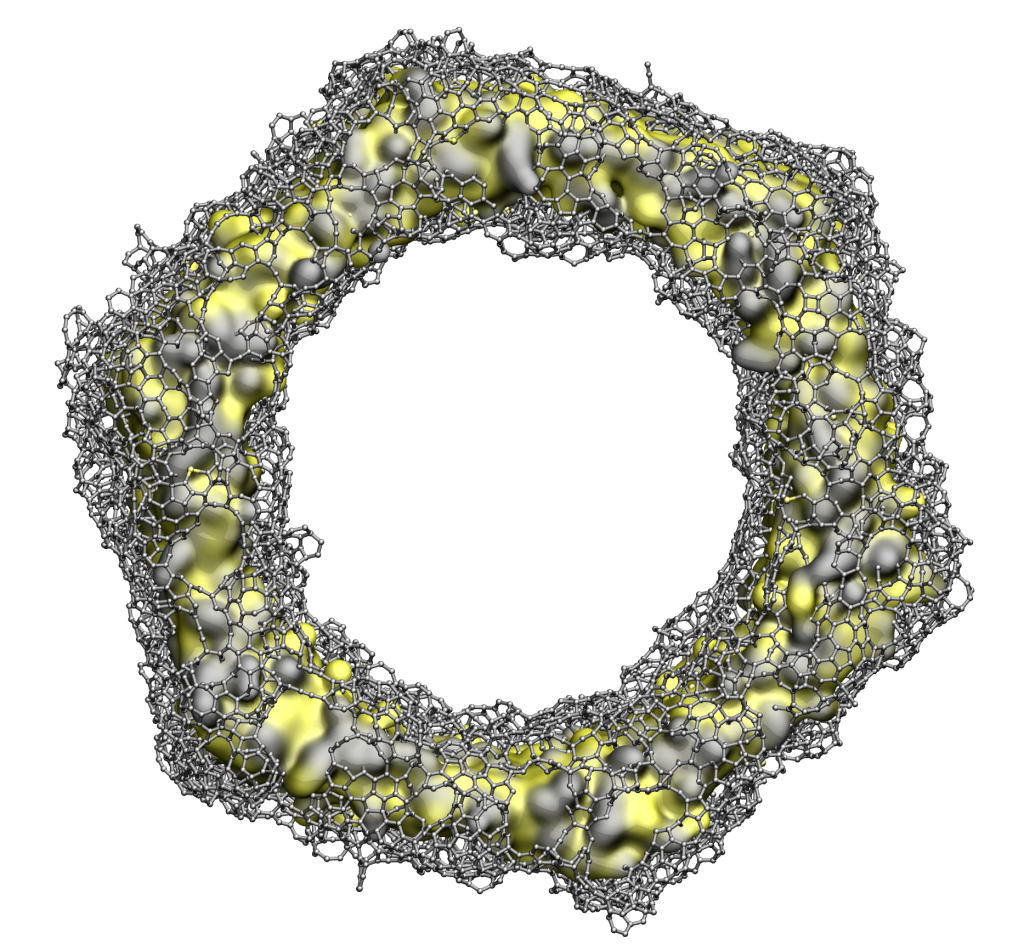
To better understand the structure at the atomistic level, several in silico models were generated using reactive molecular dynamics simulations. The simulations allowed to rationalize the processes at the atomic level and confirmed the formation of a graphene layer on several exposed SiC surfaces. The structures were characterized and used to study their chemical properties as their propensity to bond hydrogen.
| People | Stefano Veronesi*, Stefan Heun, Ylea Vlamidis, Emanuele Pompei |
| Keywords | Hydrogen storage, graphene, 3D arrangement |
| Methods, techniques | STM, LEED, TDS, Raman spectroscopy, SEM. |
| Collaborations | Prof. Ulrich Schmid, Institute of Sensor and Actuator Systems, TU Wien, Vienna, 1040, Austria Prof. Ariel Ismach, Department of Materials Science and Engineering, Tel Aviv University, Ramat Aviv, Tel Aviv, 6997801, Israel Prof. Sara Bals, EMAT, University of Antwerp, Groenenborgerlaan 171, Antwerp, B-2020, Belgium |
| Publications | |
| S. Veronesi, G. Pfusterschmied, F. Fabbri, M. Leitgeb, O. Arif, D.A. Esteban, S. Bals, U. Schmid, S. Heun, 3D arrangement of epitaxial graphene conformally grown on porousified crystalline SiC Carbon 2022 | |
| A. Macili, Y. Vlamidis, G. Pfusterschmied, M. Leitgeb, U. Schmid, S. Heun, S. Veronesi, Study of hydrogen absorption in a novel three-dimensional graphene structure: towards hydrogen storage applications, Applied Surface Science 2023 |